Guide To High Performance Austenitic Stainless Steel -- Metallurgical Overview
Feb 02, 2023
1. Types of stainless steel
Stainless steel is an iron-based alloy with a chromium content of not less than 10.5%. It is widely used because of its good corrosion resistance and high temperature performance. When the chromium content reaches 10.5%, a layer of chromium-rich oxide will be formed on the surface of steel, which is called passivation layer or passivation film. This film protects stainless steel from rusting like ordinary steel. There are many kinds of stainless steel, but all stainless steel should meet the minimum chromium content requirement.
Stainless steel is divided into five categories: austenitic stainless steel, ferritic stainless steel, duplex stainless steel (with mixed structure of ferrite and austenite), martensitic stainless steel and precipitation hardening stainless steel. The classification of these categories is related to the crystal structure (atomic arrangement) and heat treatment of stainless steel. A group of crystals with the same crystal structure in a metal is called a phase. There are three main phases in stainless steel: austenite, ferrite and martensite. The type and quantity of metallographic structure of stainless steel can be determined by standard metallographic inspection process and optical metallographic microscope.
The characteristic of austenitic stainless steel is that the metallographic structure is mainly austenitic. The crystal structure of austenite phase is face-centered cubic (fcc) structure, that is, there is an atom at each corner and center of each face of the cube. In contrast, the crystal structure of ferrite phase is body-centered cubic (bcc) structure, with one atom at each corner and center of the cube. The crystal structure of martensite phase is high strain body-centered tetragonal structure.
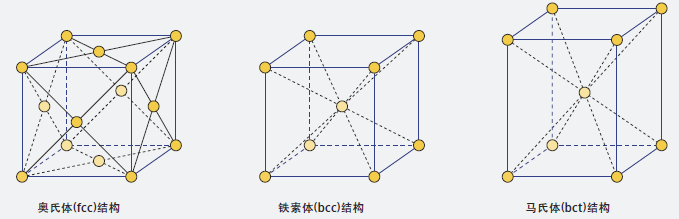
The crystal structure of austenite phase is face-centered cubic (fcc) lattice, ferrite phase is body-centered cubic (bcc) lattice, and martensite phase is body-centered tetragonal (bct) lattice.
1.1 Austenitic stainless steel:
Austenitic stainless steel has no magnetism, medium yield strength, high work hardening rate, high tensile strength, good plasticity and excellent low-temperature toughness. Unlike other stainless steels, the toughness of austenitic stainless steels decreases slowly with the decrease of temperature. Austenitic stainless steel has no definite ductile-brittle transition temperature (DBTT), so it is an ideal material for low temperature applications.

Diagram of ductile-brittle transition temperature (DBTT) of austenitic, ferritic and duplex (austenitic-ferritic) stainless steel. The actual DBTT depends on the section thickness, chemical composition and grain size. The DBTT of ferritic stainless steel is generally 20 to - 30 ° C (70 to - 22 ° F).
Austenitic stainless steel has good weldability and can be made into various complex shapes. This series of stainless steels cannot be hardened or strengthened by heat treatment, but can be strengthened by cold forming or work hardening (see ASTM A666). Austenitic stainless steel, especially standard austenitic stainless steel, has a potential disadvantage, that is, compared with ferritic stainless steel and duplex stainless steel, it is prone to chloride stress corrosion cracking.
300 series or standard austenitic stainless steel generally contains 8%~11% nickel and 16%~20% chromium. The metallographic structure of standard austenitic stainless steel is mainly composed of austenitic grains and contains a small amount (generally 1~5%) of δ Ferrite phase (Fig. 3). Because of the presence of ferrite phase, these austenitic stainless steels have a little magnetism.

The typical metallographic structure of forged stainless steel 304L is composed of austenitic grains and individual strip ferrite © TMR Stainless.
Compared with 300 series stainless steel, 200 series austenitic stainless steel has lower Ni content, but higher Mn and N content. The strength and strain hardening coefficient of 200 series stainless steel are higher than that of 300 series stainless steel. Because of the low nickel content, 200 series stainless steel is sometimes used as a cheap substitute for 300 series stainless steel.
The microstructure of high performance austenitic stainless steel is all austenitic phase without ferromagnetism (Fig. 4). Compared with standard austenitic stainless steel, high-performance austenitic stainless steel contains more nickel, chromium and molybdenum elements, and generally contains nitrogen. These stainless steels have strong corrosion resistance in corrosive environments such as strong acid, strong alkali and high chloride media, including brackish water, seawater and salt water. Compared with standard austenitic stainless steel, high-performance austenitic stainless steel has higher strength grade and better resistance to stress corrosion cracking.

Metallographic structure of 6% Mo high performance austenitic stainless steel, all composed of austenitic grains © TMR Stainless.
1.2 Ferritic stainless steel:
The microstructure of ferritic stainless steel is ferrite phase. Ferritic stainless steel has low or no nickel content and is ferromagnetic. It cannot be hardened by heat treatment. The ferromagnetic properties of this type of stainless steel are similar to those of carbon steel. Ferritic stainless steel has good strength and resistance to chloride stress corrosion cracking is much better than standard 300 series austenitic stainless steel. However, their formability and weldability are poor. Their toughness is not as good as austenitic stainless steel, and will decrease with the increase of section thickness. With the decrease of temperature, ferritic stainless steel will show obvious ductile-brittle transition. Limited by these factors, the use of ferritic stainless steel is usually limited to products with thinner wall thickness, such as thin plates, strips and thin-walled tubes.
1.3 Duplex stainless steel:
Duplex stainless steel is composed of ferrite phase and austenite phase, each accounting for about half. Duplex stainless steel has many characteristics of austenitic and ferritic stainless steel. Although heat treatment cannot harden such steels, their yield strength is usually twice that of standard austenitic stainless steel, and their magnetic attraction is proportional to the volume fraction of ferrite phase. The duplex property of the metallographic structure of duplex stainless steel makes its resistance to stress corrosion cracking better than that of standard austenitic stainless steel.
1.4 Martensitic stainless steel:
The microstructure of martensitic stainless steel is mainly martensite, which may contain a small amount of secondary phases such as ferrite, austenite and carbide. Martensitic stainless steel is ferromagnetic and similar to carbon steel. The final hardness depends on the specific heat treatment. Martensitic stainless steel has high strength, good wear resistance, poor toughness and high ductile-brittle transition temperature. They are difficult to weld and generally require post-weld heat treatment. Therefore, martensitic stainless steel is generally limited to non-welding applications. The chromium content of martensitic stainless steel is not too high. Some chromium elements precipitate in the form of carbides, resulting in low corrosion resistance, generally lower than the standard 304/304L austenitic stainless steel. Due to its poor toughness and corrosion resistance, martensitic stainless steel is generally used for applications requiring high strength and hardness, such as tools, fasteners and shafts.
1.5 Precipitation hardened stainless steel:
Precipitation hardening (PH) stainless steel can also be strengthened by heat treatment. The basic feature of this type of stainless steel is that its partial strengthening is achieved by precipitation mechanism. Fine intermetallic precipitates are produced by aging hardening heat treatment to improve the strength. Due to high chromium content, precipitation hardening stainless steel has better corrosion resistance than martensitic stainless steel, and is suitable for high-strength applications requiring good corrosion resistance. Precipitation hardening stainless steel is mainly used for springs, fasteners, aircraft parts, shafts, gears, bellows and jet engine parts.
2. Phase composition:
The alloying elements affect the phase equilibrium relationship and have a strong influence on the stability of austenite, ferrite and martensite phases. Elements added to stainless steel can be divided into ferrite phase forming elements or austenite phase forming elements. The phase equilibrium depends on the chemical composition, annealing temperature and cooling rate of the steel. Corrosion resistance, strength, toughness, weldability and formability are all affected by phase equilibrium.
Ferrite forming elements contribute to the formation of ferrite phase, while austenite forming elements promote the formation of austenite phase. Table 3 lists common ferrite and austenite phase forming elements. The grade of stainless steel and its application determine the required phase balance. Most standard austenitic stainless steels have a small amount of ferrite phase under solution annealing. Solution annealing can improve the weldability and toughness at high temperature. However, if the content of ferrite phase is too high, other properties such as corrosion resistance and toughness will be reduced. High-performance austenitic stainless steel is designed according to all austenitic phases under solution annealing condition.
To control the phase composition of steel and thus the properties of steel, it is necessary to keep the alloy elements in equilibrium. The Schaeffler structure diagram (Fig. 5) reflects the relationship between the chemical composition of stainless steel and the expected phase structure in the solidification state, as revealed by the weld microstructure. In this way, users can predict the phase equilibrium based on the given chemical composition. Calculate the "nickel equivalent" and "chromium equivalent" from the chemical composition and draw them in the figure. The formula of common parameters of Schaeffler organization chart is as follows:
Nickel equivalent=% Ni+30% C+0.5% Mn+30% N
Chromium equivalent=% Cr+% Mo+1.5% Si+0.5% Nb
The typical high-performance austenitic stainless steel contains about 20% Cr, 6% Mo, 20% Ni and 0.2% N, which is located in the single-phase austenitic phase zone in the figure, near the "ferritic" line with nickel equivalent of about 24 and chromium equivalent of about 26. In contrast, the chemical composition of standard stainless steel (such as 304) corresponds to the duplex zone of austenite+ferrite (A+F) with a small amount of ferrite phase. Ferritic stainless steel is in the ferrite phase zone in the figure, and duplex stainless steel is in the austenite+ferrite (A+F) duplex zone.








